We use cookies on our website to improve the website and your experience.
Read moreThe Scientific Foundation of Albumedix
For information on Albumedix recombinant human albumin's visit our Products page.
Highly temperature and pH stable
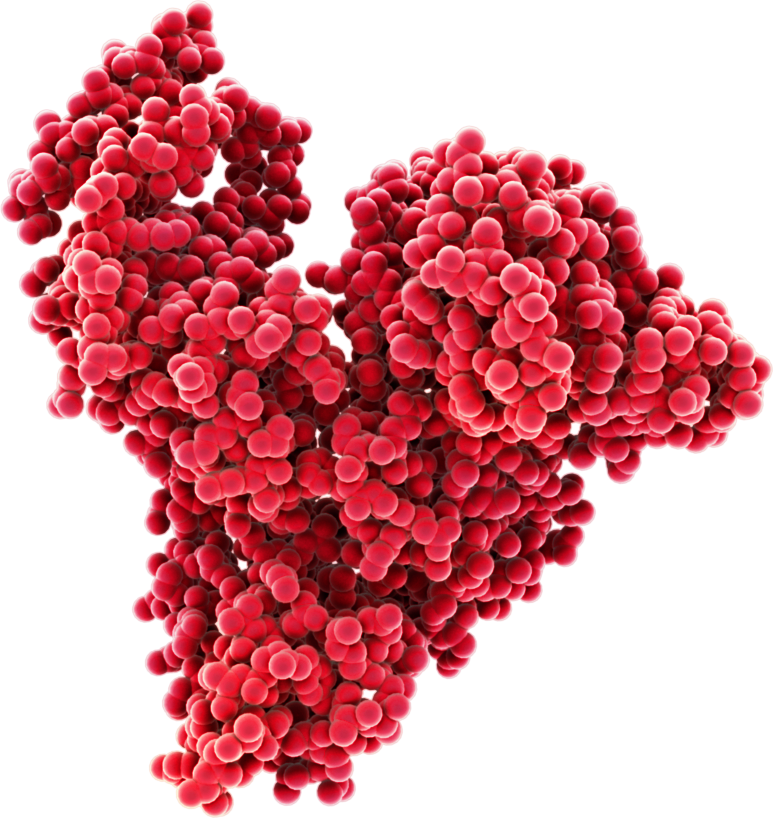
The structure of albumin gives it excellent stability, making it resilient to environmental stress both outside and inside the body.
C-terminus available for fusion of peptides
Albumin is responsible for maintaining oncotic pressure, plasma pH and distribution of a variety of molecules, making it a very well-studied protein.
Albumin covers hydrophilic and hydrophobic surfaces in a monolayer: 1-2 mg covers 1 m2
Molecular structure of albumin: 70kDa comprising two thirds alpha-helix and 17 disulphide bonds
Unlike HSA, recombinant human albumin (rAlb) products have a remarkably high and consistent quality profile.
Multiple binding sites for various compounds enable non-covalent binding and prevention of self-aggregation
Non-immunogenic
N-terminus available for fusion of peptides
Highly soluble: present at 35-45 g/L in blood
Cysteine-34 for chemical conjugation of peptides and scavenging against free radicals
Albumin – Naturally designed for stabilization and delivery of pharmaceuticals
Human serum albumin (HSA) is the most abundant protein in human blood, constituting approximately half of the total plasma proteins. Albumin is responsible for maintaining oncotic pressure, plasma pH and distribution of a variety of molecules, making it a very well-studied protein.
It is an outdated perception that the complete picture of albumin has been uncovered. With more than 30 years of experience working with albumin and its biology, we can safely say that we have only just begun to unlock the vast potential of this unique molecule and its exceptional biological relevance.
The structure of albumin gives it excellent stability, making it resilient to environmental stress both outside and inside the body.
Medical use of human serum albumin
Albumin has a long history of medical use dating from the 1940’s. Originally prepared from pooled plasma, its unique properties have been exploited in a range of medical applications including plasma volume expansion, the treatment of sepsis (read more), detoxification, imaging and diagnostics, medical device coating and therapeutic peptide and protein stabilization.
Research around the albumin molecule and its biology has increased dramatically during the past decade (read more). Several albumin binding proteins and receptors have been identified, and it has been demonstrated that these play an important role in the transport of albumin between different compartments, as well as its internalization, degradation, salvage and recycling. Most well understood is the interaction of albumin with the neonatal Fc receptor (FcRn) and the impact that this receptor has on the long serum half-life of albumin (approximately three weeks).
Request a sampleRecombinant human albumin
Driven by the desire for high quality, human- and animal-component free albumin products, structurally and functionally equivalent recombinant versions to HSA have been developed over the past decades. HSA prepared from plasma present different genetic variants and exhibit varying degrees of N-terminal truncation, glycation and oxidation due to the extracted albumins extended residence time in the body. Unlike HSA, recombinant human albumin (rAlb) products have a remarkably high and consistent quality profile.
Read more about our Recombumin® portfolio of rAlb products, formulated to maintain the unique properties of natural human albumin to enhance protein & peptide formulation, vaccine stabilization and cell therapy processing.
VISIT THE ALBUMEDIX LIBRARY
Here you will find scientific papers authored and co-authored by some of Albumedix's leading scientists as well as our collaborators and partners.
FIND ARTICLESAlbumin-enabled therapeutics
Given its intrinsic biochemical and biophysical properties, albumin has been successfully employed for many years in the development and manufacturing of biopharmaceutical drugs. Today, several albumin-enabled therapeutics are commercialized and offer game-changing treatment options to patients around the world; achievable through the therapeutically enhancing and supportive effect of albumin and pioneering albumin-based technologies.
With more than 30 years of scientific knowledge on the unique biology of albumin, and a dedication to better health, we have developed a proprietary albumin-based drug enhancing technology platform, Veltis®, for the creation of safer and more effective biotherapeutics.
Read more about our Veltis technology platform, and how partners are leveraging this technology (pipeline) in their development of differentiated biopharmaceuticals for increased therapeutic impact and ultimately improved patient quality of life.
